Phenotypic Effects Caused by the Multiple Allele Series of the dil-locus (dilute) in the Budgerigar[Melopsittacusundulatus]
MUTAVI – Research & Advice Group, The Netherlands
During the development of normally pigmented feathers, pigment cells also known as melanocytes, synthesize numerous melanosomes (pigment granules) [13], that are distributed by melanocyte dendrites into neighbouring keratinocytes. Colour mutations alter normal pigment synthesis or pigment dispersion.
One of the phenotypic effects caused by mutation is pigment dilution, a quantitative altering in pigmentation. The dil-locus (dilute) in the Budgerigar is an excellent example of this phenomenon.
Pigment dilution is caused by; reduction of the total amount of melanocytes, a reduced amount of melanosomes per melanocyte, deformed melanosomes or poor pigment dispersion.
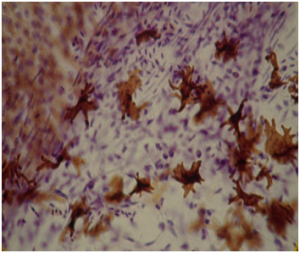
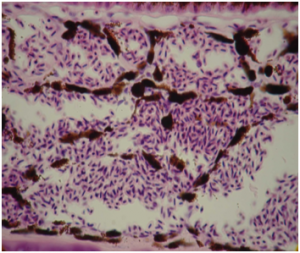
Microscopic examination of wild-type (dil +/ dil + ) and dilute (dil / dil ) melanocytes as well, showed dendrite development to be seriously affected in dilute birds. Wild-type melanocytes are characterized by large amounts of dendrites reaching within the barbules of a feather. These dendrites play an important role in dispersing melanosomes from melanocytes to keratinocytes in developing feathers. However, dilute melanocytes are often spherical-shaped and mostly lack any distinct dendrites. Sometimes dilute melanocytes show some very short and poor developed dendrites.
Therefore the lack of dendrites dramatically reduces the amount of dispersed melanosomes in feather keratinocytes. In diluted Budgerigars the amount of melanosomes in feather barbules is decreased by approximately 80%. In greywing Budgerigars the decrease is about 50%.
During pigmentsynthesis, melanosomes are removed from the cytoplasm of the melanocyte through its dendrites. The cytoplasm normally contains a moderate amount of pigment granules. Dilute melanocytes lack the mechanism to remove new developed melanosomes from their cytoplasm and eventually this will congest the entire cell. This obstruction is the indirect result of the poorly developed dendrites.
Pigment distribution in mammals can be divided into the following fases:
The introduction of dendrites, the apocapation of the top of the dendrite, withdrawal of the dendrite and the uptake of the top of the dendrite into keratinocytes allowing the dispersion of pigment granules [6]. These four steps connect the melanocyte dendrites with the neighbouring keratinocytes. This total group of cells is called the epidermal melanin unit.
The dilute factor obstructs normal dendrite development causing a separation between the keratinocytes and the source from which they obtain their pigment granules. This will result in dysfunction of the epidermal melanin unit.
Several pigment dilution mutants have been reported in Budgerigars e.g. (German) bronze fallow (a bz), the (English) pale fallow (pf), the (Scottish) plum eyed fallow (pl) and the faded (fa). However, the ethiology of these mutations is quite different from the dilute factor. In these four phenotypes we have to deal with a qualitative altering of pigment unlike the multiple allele series of the dil-locus causing a quantitative decrease of pigment granules. Colordepth is not only determined by the presence or absence of the darkfactor (D) but also depends on the amount of dispersed melanosomes as well as pigment production itself.
Investigations on the ultrastructural level (electron microscope) showed microtubules (long unbranched, hollow cylinders) ordered in length, are prominent structures in melanocyte dendrites. This has been found in melanocytes of the fowl [2]. Tylney [15] provided evidence that microtubules are responsible for cel-asymmetry. They are part of profound cel-skeleton elements like dendrites. Therefore it could be possible that the dil-locus in the Budgerigar affects the synthesis of these microtubules and in this way dramatically obstructs the development of melanocyte dendrites.
Other pigment diluting mutants have been found e.g. the sex-linked pastel factor in canaries [10]. Mr. Kop (1985) found during microscopical examination of sex-linked pastel melanocytes in the canary [Serinuscanariacanaria] an almost unhibited pigment granule production. These melanocytes were unable to disperse their pigment granules into neighbouring keratinocytes. The result of this event in canaries is self-destruction of the melanocytes and even sometimes transfers of totally congested melanocytes into feather barbs. Mr. Kop also described adendritic melanocytes in opal (dilute) canaries [8,9,11] an autosomal recessive mutant also causing obstruction of pigment dispersion.
Ludwig Auber [1] reported the incidental uptake of adendritic melanocytes in the medulla of developing barbs from wingcovert feathers in dilute Budgerigars. My microscopically examinations of dilute wingcovert feathers showed pigment clusters and a large amount of macro-melanosomes [7,12]. Obviously these “melanin macroglobules”, as they are referred to in science nowadays [11], develop during the obstruction of pigment dispersion.
These giant pigment granules [12] sometimes are 500 times larger than a normal pigment granule (melanosome) [7,12] and in some cases could fill up the whole medulla. It is particularly the presence of these macro-melanosomes that prevents clearwings from having real clear wings.
Explanation of symbols.
Multiple allele series are written with one basic symbol for the whole series, the individual alleles are designated with a superscript.
Multiple allele series of the dil-locus in the Budgerigar.
Phenotypes:
dil+/ dil + = Wildtype
dil gw/ dil cw = FBC greywing
dil cw / dil cw = Clearwing
dil gw / dil gw = Greywing
dil / dil = Dilute
All presented in homozygous state except for the FBC greywing which is the result of the interaction betweenthe dil gw and dil cw alleles.
Genotypes:
dil gw / dil cw = FBC greywing
dil gw / dil = Greywing / dilute
dil gw / dil gw = Greywing
dil cw / dil cw = Clearwing
dil cw / dil = Clearwing / dilute
dil / dil = Dilute
Order of dominance:
dil +>dil gw<>dil cw>dil
Allele symbols:
gw = greywing
cw = clearwing
In this multiple allele series we recognize four mutant phenotypes; the FBC greywing, the clearwing, the (intermediate) greywing and the dilute. Genetically six different genotypes have been designated.
An FBC greywing can only be split for clearwing, a clearwing can only be split for dilute.
The formula dil gw / dil cw demands an explanation. A bird having this genotype shows the FBC greywing phenotype because the full bodycolour of the clearwing dominates the diluted bodycolour of the intermediate greywing, on the other hand the grey wings of the latter mentioned dominates the “white” or “yellow” wingcolour of the clearwing. This is not surprizing because multiple alleles always act additionally towards one another [4,14].
In this multiple allele series we have to deal at least with three mutant alleles causing six different genotypes. We are still searching for evidence that there possibly is a fourth mutant allele at this locus. This could possibly explain the great number of colourshades that have been reported from time to time. If there is a fourth allele all possible genotypes would have been 10, if there were five there would be 15, if there were six there would be 21 etc.etc.
The dil-locus in the Budgerigar is an excellent example for demonstrating the order of dominance between gene pairs in a series of multiple alleles.
Summary
The genetic basis and gene action of the dil-locus (dilute) , an autosomal recessive pigment-diluting mutant in the Budgerigar, has been discussed. Examination of feather and eye tissue with the light microscope showed that dilute obstructs melanocyte dendrite development. Thus very few pigment granules are dispersed into neighbouring feather keratinocytes. The lack of dispersal causes the dilute phenotype and the melanocytes themselves become congested with pigment granules. In this multiple allele series we recognize at least three different phenotypes and six different genotypes. The possible presence of a fourth allele is proposed although we are still waiting for evidence.
Consulted and cited literature:
[1] Auber L., (1941) The Colours of Feathers and their Structural Causes in Varieties of the Budgerigar, Melopsittacusundulatus [Shaw] Thesis: University of Edinburg.
[2] Brumbaugh J.A., Chatterjee G., Hollander W.F., (1972) Adendritic Melanocytes: A Mutation in Linkage Group II of the Fowl. Journal of Heredity Vol.63: p.p.19-25
[3] Brumbaugh J.A., Hollander W.F., (1966) Genetics of Buff and Related Color Patterns in the Fowl. Poultry Science Vol.45: p.p.451-457
[4] Carlson E.A., (1959) Comparative Genetics of Complex Loci Quart.Rev.Biol: p.p.33-67
[5] Hollander W.F., Walther P.L., (1962) Recessive “Lavender” in the Muscovy Duck. Journal of Heredity Vol.53: p.p.81-83
[6] Klaus S.N., (1969) Pigment Transfer in Mammalian Epidermis. Arch.Derm. Vol.100: p.p.756-762
[7] Konrad K., Wolff K., Honigsmann H., (1974) The Giant melanosome: a Model of Deranged Melanosome-morphogenesis. Journ.Ultrastr.Research Vol.48: p.p.102-123
[8] Kop F.H.M., (1983) Opaalfactor: GeenStructuurfactor maar Melanocytenfactor. De Vogelwereldjaargang 38: p.p.558-564
[9] Kop F.H.M., (1987) De Opaalfactor ONZE VOGELS no.4: p.p.170-171
[10]Kop F.H.M., (1985) Het Kweken van Kanaries ZuidBoekproductiesb.v. (VolièreVademecum)
[11]Nakagawa H., Hori Y., Sato S., (1984) The nature and origin of the Melanin Macroglobule. Journal of Invest.Derm. Vol.83 no.2: p.p.134-139
[12]Onsman I., (1990) Erfelijkheid, vederstructuurenpigmentatie van diverse mutatiesen mutatie-combinatiesbij de Grasparkiet (Melopsittacusundulatus). Syllabus Grasparkieten Symposium: p.p.20-25
[13]Onsman I., (1987) Terminologietegebruikenbijmelaninebevattendecellenen pigmentvorming. ONZE VOGELS no.5: p.p.218-219
[14]Stern C., (1930) Über die additive WirkungMultipler Allele. MultipeleAllelie: p.p.261-290
[15]Tilney L.G., (1968) Ordening of subcellular units: The assembly of microtubules and their role in the development of cell form. Developmental Biology Suppl. 2: p.p.63-102
[author title=”By: Inte Onsman, Research Coordinator” author_id=””]